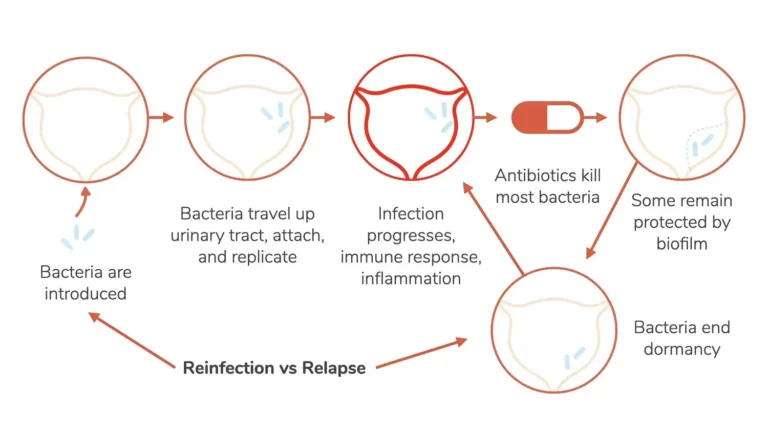
SCIENCE OF UTI'S AND RELATED CANCERS
Why are UTI's so important?
“Urinary tract infection (UTI) is one of the most common infectious diseases and the leading cause of both community-acquired and healthcare-associated infections that develop during medical care. Annually, UTIs affect around 30 million people in the United States, making them the single greatest cause of antibiotic use outside hospital settings [134].”
Prevalence and Gender Disparity
Women are disproportionately affected by UTIs, with 60% of women in the U.S. experiencing a UTI in their lifetime, compared to just 14% of men [33]. However, these statistics are likely underestimated, as approximately 50% of UTIs do not come to medical attention.
The increased risk for women is partly due to anatomical factors. The female urethra’s proximity to the vagina and rectum can facilitate the unintentional introduction of pathogenic bacteria, such as Escherichia coli. This bacterium, the most common pathogen associated with acute and recurrent UTIs in women, can migrate from the rectum to the genital area and ascend the urethra to the bladder.
Infections that spread to the kidneys or bloodstream pose more significant health risks and are harder to treat. Lower urinary tract infections often present as cystitis or prostatitis, while upper UTIs can result in pyelonephritis. Among otherwise healthy, sexually active women over 18 years, UTIs tend to occur approximately every other year [34].
The prevalence of UTIs increases with age, often due to functional and anatomical changes [32]. Among men aged 50 to 80, up to 90% experience troublesome lower urinary tract symptoms [83]. A population-based study of 31,000 residents in Calgary found that UTI incidence among women peaks in their twenties (30 per 1,000), decreases slightly during later reproductive years, and then steadily increases, reaching a maximum of 125 per 1,000 among those aged 80 and older [35].
Recurrent UTIs and Antibiotic Resistance
Recurrent UTIs are defined as two or more infections within six months or three or more within 12 months. These differ from single instances, as they are often resistant to one or more antibiotics. People with weakened immune systems or chronic conditions are particularly vulnerable to antibiotic-resistant infections [39, 73].
The overuse of beta-lactam antibiotics (e.g., penicillins, cephalosporins, monobactams, carbapenems) has decreased their efficacy in eradicating vaginal colonization by E. coli. Clinically, these antibiotics are associated with rapid recurrence of UTIs after treatment compared to other agents [68, 69].
Microbiota Disruption and Pathogen Proliferation
The intestinal microbiota is essential in maintaining mucosal protection across various organs. When disrupted, it can lead to UTI pathogenesis and recurrence [41]. Changes in the microbiota increase pathogen colonization of the vagina and urethra, allowing bacteria to ascend to the bladder and kidneys.
Repeated antibiotic use reduces bacterial diversity and disrupts the inflammatory process, enabling pathogens like Proteus, Staphylococcus, Pseudomonas, Providencia, Enterococcus, Enterobacter, Ureaplasma, and Klebsiella to proliferate [24-26]. These pathogens increase proinflammatory protein production (e.g., cytokines) and suppress anti-inflammatory responses [67]. This imbalance interferes with iron homeostasis and oxygen delivery to tissues, fostering an environment where bacteria thrive [77-81].
Some pathogens also deplete citrate, which helps prevent kidney and bladder stones. They produce urease and other enzymes that generate ammonia, carbon dioxide, uric acid, and free radicals, promoting biofilm formation and enhancing survival under adverse conditions, such as antibiotic exposure or nutrient deprivation [29].
Association with Cancer and Other Health Risks
Recurrent UTIs are strongly linked to higher incidences of acute urinary retention and various cancers, including bladder, kidney, male colorectal, prostate, and female liver cancers. Acute urinary retention is characterized by a sudden, painful inability to void and occurs more frequently in men, with a 2.2 to 8.8 per 1,000 incidence rate annually, increasing with age [84-88].
A 2018 Danish study involving 5.8 million residents found that the absolute risk of urinary tract cancer among patients aged 50 and older was 1.3% at three months, 1.8% at one year, and 2.5% at five years following a first diagnosis of acute non-surgical-induced urinary retention. The study reported significantly higher rates of invasive and non-invasive bladder cancers than expected in both men and women [Source].
The use of antibiotics, particularly for more than seven days, is associated with an increased risk of bladder cancer in men. Women with a history of UTI have shown a higher risk of liver cancer, particularly around 30 months after infection [91-93].
Challenges with Current Treatment Approaches
The conventional medical approach to treating UTIs has led to 53% of women with their first UTI experiencing recurrence within 6 to 12 months [94]. Men, though less prone to recurrence, may harbor bacteria within the prostate, making treatment more complex.
Antibiotics as a first-line treatment have become less attractive due to rising resistance and the associated risks of urogenital conditions and cancers [40]. The high rate of antibiotic-resistant UTIs and the associated healthcare costs, which exceeded $2 billion annually in the U.S. as of 2010, present a major public health challenge [36-38].
Furthermore, most major pharmaceutical companies have stopped investing in alternative treatments for UTIs, as the lack of profitability from antibiotic research has deterred innovation in this area.
RISK FACTORS
There are a number of anatomic and mechanical risk factors that increase the likelihood of pathogen colonization and UTIs. They include the following [95]:
- Chronic dehydration
- Diet rich in a high concentration of toxins and other non-digestible elements
- Certain forms of birth control, such as spermicidal foam and diaphragms, contain compounds that may have a toxic effect on the vaginal microbiota [70].
- Putting off urinating and defecating when you need to
- Incomplete bladder emptying
- Chronic constipation
- Atrophic vulvovaginal changes
- Lack of urinating after sex
- Cystocele in females
- Penetrative anal sex
- Prostatic hypertrophy
SOLUTIONS
Curogenix is developing simple, low-risk medications that drastically reduce the risk of recurrent UTIs and related cancers. To learn more, please refer to our About page.
There are various complementary therapies with anti-uropathogenic and bactericidal activities that are effective at treating and preventing acute and recurrent UTIs and decreasing antibiotic resistance and other urogenital diseases. They include the following [96]:
- Urinating after intercourse,
- Urinating and defecating when the urge arises
- Wiping from front to back after urination
- Vaginal estrogen in the postmenopausal woman with atrophic vulvovaginal changes. This is often an adjunctive to a prolonged course of antibiotics of 6 to 12 months [39], where the probability of a recurrent UTI within 3 months is 60% [97-99].
- Drinking between 0.5-1.0 ounces of water for each pound a person weighs daily is recommended to increase urinary volume and flush pathogenic bacteria out of the urinary tract. For example, weighing 150 pounds would require 75 to 150 ounces of water daily. Men should drink 3.7 liters of water daily, and women should have 2.7 liters. Fluids can also come in food (e.g., soups, steamed or sauteed vegetables, green smoothies, and herbal tea).
- Waiting 20 to 30 minutes before and after meals, and not during mealtimes, to drink water is recommended to prevent the dilution of digestive juices, increase the metabolism of protein, fats, and carbohydrates, and absorption of vital nutrients. This is essential to boost the immune system and bacterial diversity.
- A diet containing excess salts, sugar, chemicals, minerals (e.g., phosphorus, calcium, iron), and other toxic substances the body must excrete and interfere with the absorption of minerals (like iron and calcium) [23] in the gut, electrolytes and fluid levels, and red blood cell production. Increased binding of these items’ byproducts with calcium in the urine causes stone formation in the bladder and kidneys. Stones enable pathogens to multiply (24-26, 29], which boosts stone retention, cell injury and inflammation, and acute and recurrent UTIs [27-31, 89-90]. Iron deficiency anemia decreases the oxygen-carrying capacity of tissues [10, 15-16], alters intestinal permeability, damages the mucosa, lowers bacterial diversity, and modulates the inflammatory process. Stimulating a high loss of protein and premature cell death enables the multiplication of pathogens in the GI and GU tracts, which correlates with acute and recurrent infections, various inflammatory chronic conditions, and cancer growth [14, 18-21]. The top insulters include:
- Animal products (like red meat, dairy products, eggs, poultry, shellfish, and fish)
- High phosphorus items (like additives, preservatives, and artificial sweeteners – commonly found in canned, frozen, processed, and fast foods; bottled colas, condiments, ultra-sweet fresh and dried fruits such as dates, berries, and grapes
- Oxalate-rich foods [like spinach, starchy grains (e.g., soybeans, tofu, wheat, and bran cereals), nuts, root vegetables (e.g., potatoes, sweet potatoes, beets, carrots)]
- Polyphenol-rich items such as ultra-sweet fresh and dried fruits such as berries, grapes, dates, alcohol, coffee, cocoa, nuts, olives, black and green tea, spinach, and artichokes.
Our Solutions
Medical Consultations
Virtual telemedicine session with a UTI specialist to alleviate pain and treat various physical, mental, and emotional conditions.
Treatments
Medications specifically designed to remedy UTIs, protect from recurrent UTIs and associated cancers, and nurture the microbiota and overall health.
SOURCES
- Ramsay DJ. Homeostatic control of water balance. In: Arnaud MJ, editor. Hydration Throughout Life. Montrouge: John Libbey Eurotext; 1998. pp. 9–18.
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15(10):630–8. [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. [PubMed] [Google Scholar]
- McCranor BJ, Kim MJ, Cruz NM, et al. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis. 2014;52:126–133.
- Gifford GE, Duckworth DH. Introduction to TNF and related lymphokines. Biotherapy. 1991;3:103–111.
- Ravasi G, Pelucchi S, Greni F, et al. Circulating factors are involved in hypoxia-induced hepcidin suppression. Blood Cells Mol Dis. 2014;53:204–210.
- Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463.
- Raj DS. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38:382–388.
- Levy-Costa R.B., Monteiro C.A. Cow’s milk consumption and childhood anemia in the city of São Paulo, southern Brazil. Rev. Saúde Pública. 2004;38:1–6.
- Ziegler E.E. Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr. Rev. 2011;69:37–42. doi: 10.1111/j.1753-4887.2011.00431.x.
- Benkhedda K.; L’abbé M. R.; Cockell K. A. Effect of Calcium on Iron Absorption in Women with Marginal Iron Status. Br. J. Nutr. 2010, 103 (5), 742–748. 10.1017/S0007114509992418.
- Bondi S.A., Lieuw K. Excessive Cow’s Milk Consumption and Iron Deficiency in Toddlers, Two Unusual Presentations and Review. ICAN Infant Child Adolesc. Nutr. 2009;1:133–139. doi: 10.1177/1941406409335481. [CrossRef] [Google Scholar]
- Mantadakis E., Zikidou P., Tsouvala E., Thomaidis S., Chatzimichael A. Severe iron deficiency anemia and anasarca edema due to excessive cow’s milk intake. Turk. J. Pediatrics. 2019;61:102–106. doi: 10.24953/turkjped.2019.01.016.
- Braamskamp M.J.A.M., Dolman K.M., Merit M., Tabbers M.M. Clinical practice. Protein-losing enteropathy in children. Eur. J. Pediatrics. 2010;169:1179–1185. doi: 10.1007/s00431-010-1235-2.
- Nairz M., Theurl I., Wolf D., Weiss G. Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wien. Med. Wochenschr. 2016;166:411–423. doi: 10.1007/s10354-016-0505-7
- Hurrell R. F.; Reddy M.; Cook J. D. Inhibition of Non-Haem Iron Absorption in Man by Polyphenolic-Containing Beverages. Br. J. Nutr. 1999, 81 (4), 289–295. 10.1017/S0007114599000537.
- Petry N.; Egli I.; Zeder C.; Walczyk T.; Hurrell R. The Journal of Nutrition Nutrient Physiology, Metabolism, and Nutrient-Nutrient Interactions Polyphenols and Phytic Acid Contribute to the Low Iron Bioavailability from Common Beans in Young Women 1,2. J. Nutr. 2010, 140, 1977–1982. 10.3945/jn.110.125369.
- Me N.; Faizadatul N.; Aw A. Determination of Phytate, Iron, Zinc, Calcium Contents and Their Molar Ratios in Commonly Consumed Raw and Prepared Food in Malaysia. Mal. J. Nutr. 2009, 15 (2), 213–222.
- Harland B. F.; Morris E. R. Phytate: A Good or a Bad Food Component?. Nutr. Res. (N.Y.) 1995, 15 (5), 733–754. 10.1016/0271-5317(95)00040-P.
- Wilson M. S. C.; Bulley S. J.; Pisani F.; Irvine R. F.; Saiardi A. A Novel Method for the Purification of Inositol Phosphates from Biological Samples Reveals That No Phytate Is Present in Human Plasma or Urine. Open Biol. 2015, 5 (3), 150014. 10.1098/rsob.150014.
- Brouns F. Phytic Acid and Whole Grains for Health Controversy. Nutrients 2022, 14 (1), 25. 10.3390/nu14010025.
- Cook J. D.; Monsen E. R. Food Iron Absorption in Human Subjects. III. Comparison of the Effect of Animal Proteins on Nonheme Iron Absorption. Am. J. Clin. Nutr. 1976, 29 (8), 859–867. 10.1093/ajcn/29.8.859. [PubMed] [CrossRef] [Google Scholar]
- Schaffer JN, Pearson MM. Proteus mirabilis and urinary tract infections. Urin Tract Infect Mol Pathog Clin Manag. 2016;383–433.
- Kokkayil P, Dhawan B. Ureaplasma: current perspectives. Indian J Med Microbiol. 2015;33(2).
- Flannigan R, Choy WH, Chew B, Lange D. Renal struvite stones – pathogenesis, microbiology, and management strategies. Nat Rev Urol. 2014;11(6):333–341. doi: 10.1038/nrurol.2014.99. [PubMed]
- Tavichakorntrakool R, Prasongwattana V, Sungkeeree S, Saisud P, Sribenjalux P, Pimratana C, et al. Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol Dial Transplant. 2012;27(11):4125–4130. doi: 10.1093/ndt/gfs057. [PubMed]
- Zhao Z, Zeng G. Stone removal for treating recurrent urinary tract infections in stone-formers: two birds with one stone or just too soon to tell? World J Urol. 2020;38(11):2995–6. 10.1007/s00345-019-03015-y. [PubMed]
- Marien T, Miller NL. Treatment of the infected stone. Urol Clin North Am. 2015;42(4):459–472. doi: 10.1016/j.ucl.2015.05.009. [PubMed]
- Kramer G, Klingler HC, Steiner GE. Role of bacteria in the development of kidney stones. Curr Opin Urol. 2000;10(1):35–38. doi: 10.1097/00042307-200001000-00009. [PubMed]
- Xie J, Huang JS, Huang XJ, Peng JM, Yu Z, Yuan YQ, et al. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. 2020;20(1):1–10. doi: 10.1186/s12866-020-01734-6. [PubMed]
- CDC. NAMCS and NHAMCS Web Tables. Table 1. Annual number and percent distribution of ambulatory care visits by setting type according to diagnosis group: United States, 2009-2010. 2010 http://www.cdc.gov/nchs/ahcd/web_tables.htm.
- Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–13. [PubMed]
- Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, Stergachis A, Stamm WE. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–474. [PubMed]
- Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: a population-based assessment. Infection. 2007;35:150–153. [PubMed]
- Engel JD, Schaeffer AJ. Evaluation of antimicrobial therapy for recurrent urinary tract infections in women. Urol Clin North Am. 1998;25:685–701. x. [PubMed]
- Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. [PubMed]
- Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–581. [PubMed]
- Hooton TM, Gupta K. UpToDate. Waltham, MA: 2016. [accessed 5 June 2016]. Recurrent urinary tract infection in women.
- Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin North Am. 2014;28:49–59. [PubMed]
- Czaja CA, Stamm WE, Stapleton AE, Roberts PL, Hawn TR, Scholes D, Samadpour M, Hultgren SJ, Hooton TM. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J Infect Dis. 2009;200:528–536. [PubMed]
- Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–445. [PubMed]
- Beerepoot M, Geerlings S. Non-antibiotic prophylaxis for urinary tract infections. Pathogens. 2016;5:5. [PubMed]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. [PMC free article] [PubMed]
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–1956. [PubMed] [Google Scholar]
- Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. [PMC free article] [PubMed]
- Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2:543–546. [PubMed] [Google Scholar]
- Herthelius M, Gorbach SL, Möllby R, Nord CE, Pettersson L, Winberg J. Elimination of vaginal colonization with Escherichia coli by administration of indigenous flora. Infect Immun. 1989;57:2447–2451. [PMC free article] [PubMed] [Google Scholar]
- McGroarty JA, Reid G. Detection of a Lactobacillus substance that inhibits Escherichia coli. Can J Microbiol. 1988;34:974–978. [PubMed] [Google Scholar]
- McGroarty JA, Tomeczek L, Pond DG, Reid G, Bruce AW. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J Infect Dis. 1992;165:1142–1144. [PubMed] [Google Scholar]
- Osset J, Bartolomé RM, García E, Andreu A. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183:485–491. [PubMed] [Google Scholar]
- Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J Urol. 1987;138:330–335. [PubMed] [Google Scholar]
- Reid G, Heinemann C, Velraeds M, van der Mei HC, Busscher HJ. Biosurfactants produced by Lactobacillus. Methods Enzymol. 1999;310:426–433. [PubMed] [Google Scholar]
- Stamey TA, Kaufman MF. Studies of introital colonization in women with recurrent urinary infections. II. A comparison of growth in normal vaginal fluid of common versus uncommon serogroups of Escherichia coli. J Urol. 1975;114:264–267. [PubMed] [Google Scholar]
- Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, Holmes KK. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–1063. [PubMed] [Google Scholar]
- Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. [PubMed] [Google Scholar]
- Schwebke JR. Role of vaginal flora as a barrier to HIV acquisition. Curr Infect Dis Rep. 2001;3:152–155. [PubMed] [Google Scholar]
- Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. [PubMed] [Google Scholar]
- van De Wijgert JH, Mason PR, Gwanzura L, Mbizvo MT, Chirenje ZM, Iliff V, Shiboski S, Padian NS. Intravaginal practices, vaginal flora disturbances, and acquisition of sexually transmitted diseases in Zimbabwean women. J Infect Dis. 2000;181:587–594. [PubMed] [Google Scholar]
- Zheng HY, Alcorn TM, Cohen MS. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis. 1994;170:1209–1215. [PubMed]
- Raz R. Urinary tract infection in postmenopausal women. Korean J Urol. 2011;52:801–808. [PMC free article] [PubMed] [Google Scholar]
- Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(Suppl 1):S74–S76. [PubMed] [Google Scholar]
- Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–756. [PubMed] [Google Scholar]
- Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, De Seta F, Quadrifoglio F, de Aloysio D, Guaschino S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol. 2002;40:2147–2152. [PMC free article] [PubMed]
- Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42–50. [PubMed] [Google Scholar]
- Pabich WL, Fihn SD, Stamm WE, Scholes D, Boyko EJ, Gupta K. Prevalence and determinants of vaginal flora alterations in postmenopausal women. J Infect Dis. 2003;188:1054–1058. [PubMed] [Google Scholar]
- Smith HS, Hughes JP, Hooton TM, Roberts P, Scholes D, Stergachis A, Stapleton A, Stamm WE. Antecedent antimicrobial use increases the risk of uncomplicated cystitis in young women. Clin Infect Dis. 1997;25:63–68. [PubMed]
- Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA. 2005;293:949–955. [PubMed]
- Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA. 2012;307:583–589. [PubMed]
- Hooton TM, Fennell CL, Clark AM, Stamm WE. Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis. 1991;164:1216–1219. [PubMed]
- Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol. 2001;30:49–52. [PubMed]
- Hutt P, Shchepetova J, Loivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero and uropathogens. J Appl Microbiol. 2006;100(6):1324–1332. [PubMed]
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013;3:a010306. [PubMed]
- Das S, Naik P, Panda P. Effect of Hemidesmus indicus R.Br. root extract on urinary tract infection causing bacteria. Int J Herbal Med. 2017;5(5):160–168.
- Das S, Panigrahi S, Panda P. Antiurobacterial activity of Punica granatum L. seed extract. European J Med Plants. 2018;22(2):1–12.
- Das S, Sahoo KR, Parida B. Bactericidal activity of Hemidesmus indicus R.Br. root extract against clinically isolated uropathogens. J Med Plant Studies. 2018;6(6):180–192.
- Moldawer LL, Marano MA, Wei H, et al. Cachectin/tumor necrosis factor-alpha alters red blood cell kinetics and induces anemia in vivo. FASEB J. 1989;3:1637–1643.
- McCranor BJ, Kim MJ, Cruz NM, et al. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis. 2014;52:126–133.
- Poggiali E, Migone De Amicis M, Motta I. Anemia of chronic disease: a unique defect of iron recycling for many different chronic diseases. Eur J Intern Med. 2014;25:12–17.
- Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–693.
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023.
- Nicolle L.E. Urinary tract infection. Crit. Care Clin. 2013;29:699–715. doi: 10.1016/j.ccc.2013.03.014. [PubMed]
- Rowe T.A., Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect. Disease Clin. N. Am. 2014;28:75–89. doi: 10.1016/j.idc.2013.10.004. [PubMed]
- Thomas K, Chow K, Kirby RS. Acute urinary retention: a review of the etiology and management. Prostate Cancer Prostatic Dis2004;7:32-7. doi:10.1038/sj.pcan.4500700. Pmid:14999235. [PubMed]
- Thorne MB, Geraci S. Acute urinary retention in elderly men. Am JMed2009;122:815-9. doi:10.1016/j.amjmed.2009.05.009. Pmid:19699373 [PubMed]
- Oelke M, Speakman MJ, Desgrandchamps F, Mamoulakis C. Acute Urinary Retention Rates in the General Male Population and in Adult Men With Lower Urinary Tract Symptoms Participating in Pharmacotherapy Trials: A Literature Review. Urology2015;86:654-65. doi:10.1016/j.urology.2015.06.025. Pmid:26142712 [PubMed]
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol1997;158:481-7. doi:10.1016/S0022-5347(01)64508-7 pmid:9224329 [PubMed]
- Klarskov P, Andersen JT, Asmussen CF, et al. Acute urinary retention in women: a prospective study of 18 consecutive cases. Scand J Urol Nephrol1987;21:29-31. doi:10.3109/00365598709180286. Pmid:3589520 [PubMed]
- Billet M, Windsor TA. Urinary Retention. Emerg Med Clin North Am2019;37:649-60. doi:10.1016/j.emc.2019.07.005. Pmid:31563200 [PubMed]
- Choong S, Emberton M. Acute urinary retention. BJU Int2000;85:186-201. doi:10.1046/j.1464-410x.2000.00409.x pmid:10671867[PubMed]
- Maj M., Bajek A., Nalejska E., Porowinska D., Kloskowski T., Gackowska L., Drewa T. Influence of mesenchymal stem cells conditioned media on proliferation of urinary tract cancer cell lines and their sensitivity to ciprofloxacin. J. Cell. Biochem. 2017;118:1361–1368. doi: 10.1002/jcb.25794. [PubMed]
- Batalha P.N., Vieira de Souza M.C., Pena-Cabrera E., Cruz D.C., da Costa Santos Boechat F. Quinolones in the search for new anticancer agents. Curr. Pharm. Des. 2016;22:6009–6020. doi: 10.2174/1381612822666160715115025. [PubMed]
- Ferraz R., Costa-Rodrigues J., Fernandes M.H., Santos M.M., Marrucho I.M., Rebelo L.P., Prudencio C., Noronha J.P., Petrovski Z., Branco L.C. Antitumor activity of ionic liquids based on ampicillin. ChemMedChem. 2015;10:1480–1483. doi: 10.1002/cmdc.201500142. [PubMed]
- Ikaheimo R, Siitonen A, Heiskanen T, Karkkainen U, Kuosmanen P, Lipponen P, Makelaph. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. ClinInfect Dis 1996; 22: 91-99.
- Rakel D. Integrative Medicine. 3rd ed. Philadelphia: Elsevier Saunders; 2012.
- Genitourinary disorders. Natural Medicines Comprehensive Database website; 2014. https://naturalmedicines.therapeuticresearch.com/.
- Nickel JC. Practical management of recurrent urinary tract infections in premenopausal women. Rev Urol 2005; 7:11-17.
- Gupta K, Stamm WE. Pathogenesis and management of recurrent urinary tract infecitons in women. World J Urol 1999; 17: 415-420.
- Alton G, Haslik M, Nieheus R, Fana F, Freeze HH. Direct manipulation of mannose for mammalian glycoprotein biosynthesis. Glycobiology 2001; 8:285-295.
- https://pubmed.ncbi.nlm.nih.gov/1931970/
- https://pubmed.ncbi.nlm.nih.gov/22167351/
- https://pubmed.ncbi.nlm.nih.gov/25901896/
- Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014;289(3):479-489.
- Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta 2001; 1528:116-126.
- Alton G, Hasilik M, NieheusI R, Fana F, Freeze HH. Direct manipulation of mannose for mammalian glycoprotein biosynthesis. Glycobiology 2001; 8:285-295.
- Sharon N. Carboydrates as future anti-adhesion drugs for infectious diseases Biochim Biophys Acta 2006; 1760: 527-537.
- Yarnell E. Botanical medicines for the urinary tract. World J Urol. 2002;20(5):285–293.
- Zafriri D, Ofek I, Adar R, Pocino M, Sharon N. Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eukaryotic cells. Antimicrob Agents Chemother. 1989;33:92–98.
- Rosen DA, Pinkener JS, Walker JN, Elam JS, Jones JM, Hultgren SJ. Molecular variations in Klebsiella pneumonia and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect Immun 2008; 76: 3346-3356.
- Natural Medicines in the Clinical Management of Urinary Tract Infections. Natural Medicines Comprehensive Database website.
- Yarnell E. Botanical medicines for the urinary tract. World J Urol. 2002;20(5):285–293. [PubMed]
- Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441–3. Doi: 10.1016/S0011-393X(05)80204-8.
- Ofek I, Goldhar J, Zafriri D, Lis H, Adar R, Sharon N. Anti-Escherichia coli activity of cranberry and blueberry juices. NEJM. 1991;324:1599.
- Schindler G, Patzak U, Brinkhaus B, von Niecieck A, Wittig J, Krahmer N, Glockl I, Veit M. Urinary excretion and metabolism of arbutin after oral administration of Arctostaphylos uvae ursi extract as film-coated tablets and aqueous solution in healthy humans. J Clin Pharmacol. 2002;42(8):920–927.
- Tomé D. The roles of dietary glutamate in the intestine. Ann Nutr Metab. 2018;73(Suppl 5):15–20.
- Cynober L. Metabolism of dietary glutamate in adults. Ann Nutr Metab. 2018;73(Suppl 5):5–14
- Brosnan JT. Glutamate at the interface between amino acid and carbohydrate metabolism. J Nutr. 2000;130((4S Suppl)):988S–90S.
- Maher TJ, Glaeser BS, Wurtman RJ. Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake. Am J Clin Nutr. 1984;39((5)):722–9.
- Darmaun D, Robert J, Bier D, Matthews D, Young V. Etude in vivo du métabolisme des acides aminés non essentiels par les isotopes stables: application à l’alanine, à la glutamine et au glutamate. Ann Endocrinol. 1985;46:355–6.
- Tomé D. The roles of dietary glutamate in the intestine. Ann Nutr Metab. 2018;73((Suppl 5)):15–20.
- Möhler H. Physiology and Pharmacology of the GABA System: Focus on GABA Receptors. In: Monti J.M., Pandi-Perumal S.R., Möhler H., editors. GABA and Sleep: Molecular, Functional and Clinical Aspects. Springer; Basel, Switzerland: 2010. pp. 3–23.
- Ito S. GABA and glycine in the developing brain. J. Physiol. Sci. 2016;66:375–379. doi: 10.1007/s12576-016-0442-7.
- Dong H, Wang N, Zhao L, Lu F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012591654.
- Pérez-Rubio KG, González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Espinel-Bermúdez MC. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2013;11(5):366-9.
- Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285-92.
- Yu Y, Hao G, Zhang Q, et al. Berberine induces GLP-1 secretion through activation of bitter taste receptor pathways. Biochem Pharmacol. 2015;97(2):173-7.
- Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79(6):437-46.
- Hu Y, Ehli EA, Kittelsrud J, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19(10):861-7.
- Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344-51.
- Gu S, Cao B, Sun R, et al. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Mol Biosyst. 2015;11(2):463-74.
- Pisciotta L, Bellocchio A, Bertolini S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 2012;11123.
- Li GH, Wang DL, Hu YD, et al. Berberine inhibits acute radiation intestinal syndrome in human with abdomen radiotherapy. Med Oncol. 2010;27(3):919-25.
- Goldenseal. Natural Medicines Comprehensive Database website. http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?cs=NEWORDER&s=ND&pt=100&id=943&fs=ND&searchid=48372755. Accessed June 22, 2014.
- https://www.nytimes.com/2024/04/24/health/fda-urinary-tract-infection-antibiotic.html
- https://www.webmd.com/diet/foods-high-in-oxalates
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6388119/